Cu-releasing nanoparticles induce the catalytic transamination of amino acids and GSSG under tumor microenvironment conditions
- Post by: nfp
- 04/07/2023
- Comments off
Catalytic cancer therapy exploits the tumor microenvironment (TME) to target cancer cells by leveraging molecules already present in the TME. Currently, only a few processes have been explored for cancer therapy, including glucose and glutathione oxidation, generation of reactive oxygen species (ROS), and alleviation of tumor hypoxia.
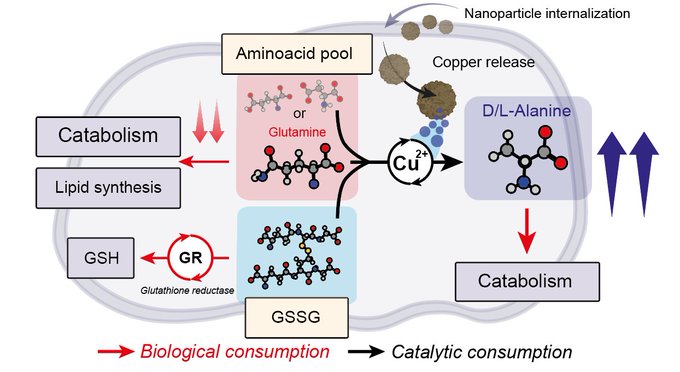
Amino groups, which are abundant in biological environments, hold promise as potential targets. Remarkably, CuFe nanoparticles exhibit catalytic activity in transamination reactions between amino acids and pyruvate, a key molecule in the TME. By reducing the available pool of amino acids, transamination disrupts cell homeostasis and effectively hinders tumor proliferation. Upon internalization of Cu-containing nanoparticles in U251-MG cells, we observed a significant and sustained decrease in glutamine and alanine levels for up to 48 hours. Furthermore, our findings indicate that not only simple amino acids but also di- and tri-peptides undergo catalytic transamination upon exposure to Cu cations released by the nanoparticles. This extends the therapeutic effects to other molecules, such as GSSG.
Mechanistic calculations revealed the formation of an imine between the oxo-group of pyruvate and the free -NH2 group of GSSG during the transamination process. The imine then coordinates with Cu(II). These results demonstrate the ability of Cu-releasing nanoparticles to catalyze transamination reactions within cells (in cellulo), thus adding a new tool to the existing arsenal of catalytic therapies.
Preprint article here.
NFP Research Area: Nanomedicine: Catalysis Against Cancer
